Current safety reporting systems cannot meet the demands of a COVID-19 response. Find out how digital transformation enables rapid understanding & management of drug safety.
What you will learn:
- Limitations of current safety reporting systems for COVID-19 treatment and vaccination initiatives
- Requirements for COVID-19 safety data capture to ensure signals are detected and assessed at the earliest opportunity
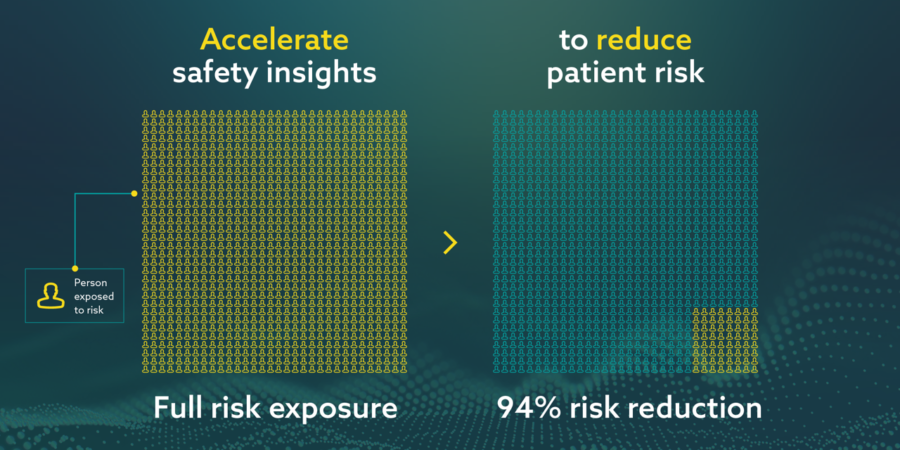
- Risk reduction in particular during the rapid exposure likely during mass vaccination campaigns
Fill out the form below to download the whitepaper: