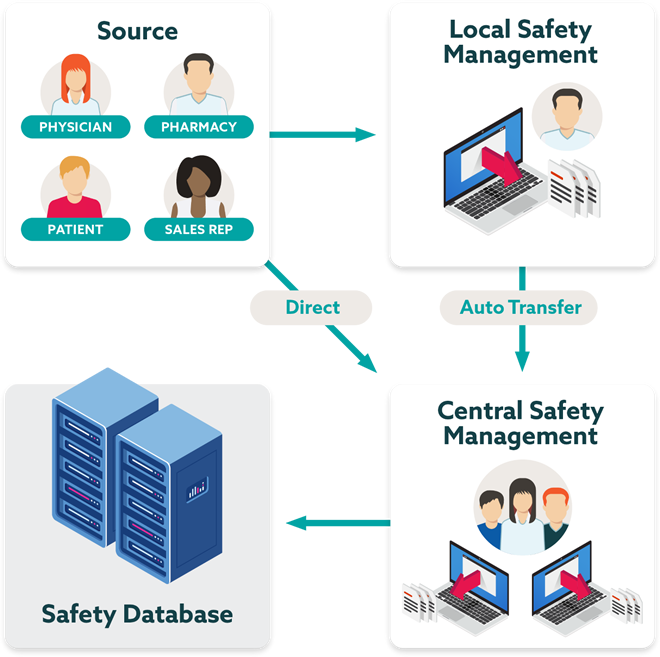
Highly scalable pharmacovigilance platform
Accelerate safety insights through a global SaaS solution that streamlines the management and understanding of safety information.
- Enhance patient engagement
- Provide a dynamic experience with standard data export
- Increase Efficiencies
- Streamline processes, automate transfer, and reduce follow up
- Simplify safety management
- Reduce case processing and management effort for vendors and safety teams